the posts in this series will be influenced by the text “Introduction to Nuclear Engineering – John R. Lamarsh“
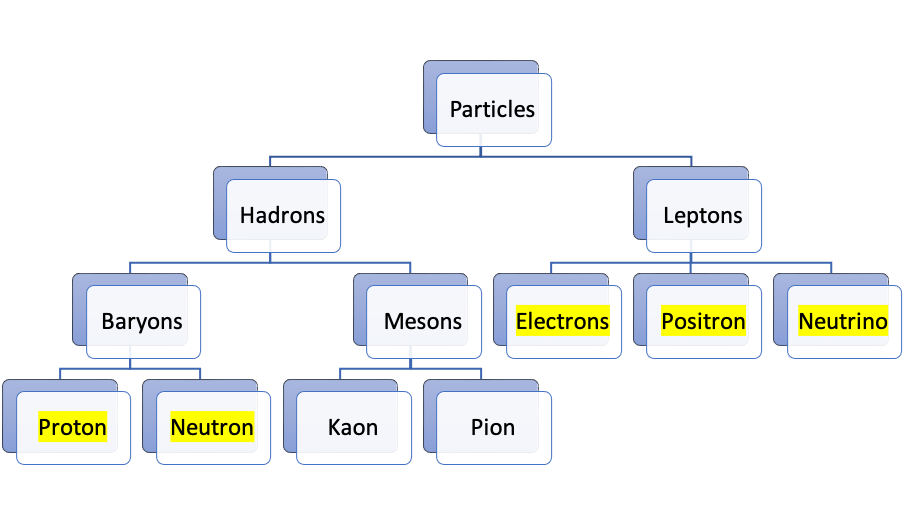
Fundamental particles are made up of quarks and held together by gluons.
the fundamental particles we’ll be interested in as nukies are electrons, positrons, neutrinos, protons and neutrons
leptrons are subject to weak nuclear forces
hadrons & baryons in particular experience weak and strong nuclear forces
hadrons are composed of quarks & exchange of gluons between quarks is responsible for the strong nuclear forces
Introduction to our beloved particles
N.B. from Einstein’s theory of relativity, mass of a particle is a function of its speed relative to the observer. when we assign masses to particles, it is important to note that the particle is at rest with respect to the observer hence “rest mass“
Electron: Rest mass, me = 9.10954 x 10-31 Kg has a charge, e = 1.60219 x 10-19 coulombs. There are two types of electrons, one with a positive charge +e and the other carries a negative charge, -e. these two particles are identical. The negative electrons, negatrons are the normal electrons, positive electrons aka positrons are relatively rare. When a positron and a negatron collide, they effectively disappear and on average, two or more photons are emitted. The process is known as electron annihilation and the photons that appear are called annihilation radiation
Proton: rest mass, mp = 1.67625 x 10-27 Kg, carries a positive charge in equal magnitude to the electron. Protons with negative charges have been found but are of no importance to us in nuclear engineering
Photon: It is a particle associated with electromagnetic waves with zero rest mass and zero charge which travels in vacuum at C i.e., speed of light = 2.9979 x 108 meters/second
Neutron: rest mass, Mn = 1.67495 x 10-27Kg - slightly larger than proton’s mass. It is electrically neutral. It is an unstable particle except when it is bound to an atomic nucleus. A free neutron decays to a proton with the emission of a negative electron (beta decay) and an anti-neutrino. The entire process takes about 12 minutes on average.
Neutrino: zero rest mass and null electrical charge. It appears in the decay (beta-plus decay) of certain nuclei. There are at least six types of neutrinos of which two are important(electron neutrinos & electron anti neutrinos). They are mostly lumped together as neutrinos.
Atomic and Nuclear structures
Atoms are the building blocks of matter. They are made up of a nucleus which are made up of protons and neutrons with a rapidly moving cloud of (negative) electrons.
The total number of protons in a nucleus is called the atomic number of the atom and is represented by the symbol, Z. change in nucleus is then +Ze
In a neutral atom with equal parts protons and neutrons, electrons are responsible for the chemical behavior of atoms and play a crucial role in the identification of chemical elements.
The number of neutrons in a nucleus is known as a neutron number and is denoted by N. The total number of nucleus i.e., protons and neutrons in a nucleus is equal to the atomic mass number or nuclear number,
Z + N = A
Species of atoms whose nuclei contain particular numbers of protons and neutrons are called nuclides which are denoted by the chemical symbol with the atomic mass number as superscript (which determines N, since N = A - Z) e.g. 4He(Z = 2) is a nuclide whose nucleus consists of z protons and neutrons. Z can be written as a subscript for greater clarity e.g.,
Atoms such as 1H and 2H whose nuclei contain the same number of protons but different numbers of neutrons i.e., same Z, different N, different A are known as Isotopes.
Oxygen has 3 stable isotopes 16O, 17O and 18O (Z = 8 N=8,9,10) and 5 unstable isotopes 13O,14O,15O,19O and 20O (Z = 8 N = 5, 6, 7, 11, 12)
Stable isotopes and some unstable ones are found naturally occurring in naturally occurring elements in nature. They’re not found in equal amounts e.g., 99.8% of naturally occurring oxygen atoms are 16O, 0.037% are 17O and 0.204% are 18O
ATOMIC AND MOLECULAR WEIGHT
Atomic weight of an atom is defined as the mass of the neutral atom relative to the mass of a neutral 12C atom on a scale in which the atomic weight of 12C is arbitrarily taken to be 12.
Let’s say:
M(AZ) – Mass of neutral atom denoted by AZ
M(12C) – Mass of neutral 12C
M(AZ) – atomic weight of AZ
Atomic weight of an element is the average atomic weight of the mixture of isotopes. If Yi is the isotopic abundance in atom percent of atomic weight, M then the atomic weight of the element is
Molecular weight is the total mass of a molecule relative to the mass of 12C
Atomic and Molecular weights are unit-less.
Gram atomic and molecular weights are defined as the amount of substance having a mass in grams according to the atomic or molecular weight of the substance this is also known as a Mole i.e., mole = atomic weight in grams
Avogadro’s law states that there must be an equal amount of molecules in equal volumes of all gases, at the same temperature and pressure.
Avogadro’s number is the number of units in one mole of any substance (defined as its molecular weight in grams) denoted by NA and is equal to 0.602214076 × 1024
From Avogadro’s number, the mass of a single atom or molecule can be calculated
e.g., for 12C
Atomic mass unit (amu) is one twelfth the mass of the neutral 12C atom
1amu = x m(12C)
m(12C) = 12amu
1amu = x 1.99268 x 10-23g =1.66057 x 10-24g
m(AZ) = x M(AZ)
m(AZ) = M(AZ)amu
Thus, the mass of any atom in amu is numerically equal to the atomic weight of the atom in question.
Atomic and nuclear radii
The average distance from the nucleus to the furthermost electron is 2 x 10-10m
The formula for the radius of a nucleus is
1fm = 10-13centimeters
R is in femtometers(fm), A – Atomic mass number
The volume of a nucleus is directly proportional to A
A/V – number of nucleons per unit volume is constant for all nuclei
Mass and Energy
From Einstein’s theory of relativity, we find out that mass and energy are equivalent and convertible, one to another. Therefore, the complete annihilation of a particle or body of rest mass Mo releases an amount of energy Erest which is gotten by,
Erest = MoC2
Where C is the speed of light. e.g., The annihilation of 1g of matter would lead to the release of E = 1 x (2.9979 x 1010)2 = 8.9874 x 1020ergs[1] = 8.9874 x 1013joules ~ 25million Kilowatt-hours.
A popular unit of energy used in nuclear engineering is the Electron-Volt i.e., eV. It is defined as the increase in the kinetic energy of an electron when it falls through an electrical potential of one volt.
1eV = 1.60219 x 10-19 coulomb x 1 volt
=1.60219 x 10-19joule
MeV = 106eV & KeV = 103eV
till next week.
this series was inspired by Mirabelle who showed me that you can just do things.
you can pick up a copy of her book here
also if you know how to properly write formulas in substack, pls hmu or drop a comment
[1] Erg is a unit of energy that is equal to 10-7 joules


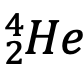
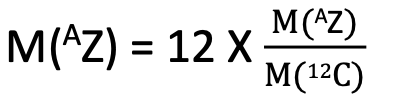
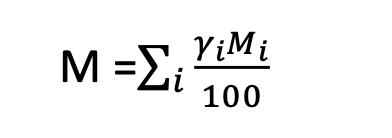
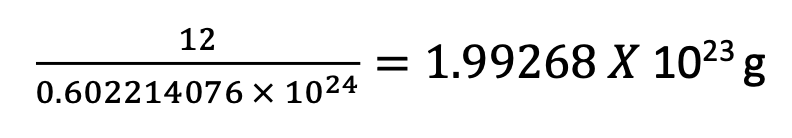

Truly, "no knowledge is ever wasted."
I may not dabble in the sciences anymore, but everything I ever learned came rushing back in an instant! This was an insightful, clear and refreshing read. Well done, Rex.
PS I'll do my best to stay humble now that I've achieved "inspirational" status 😂
Hey Rex, thank you for your post. I found it interesting especially because I know nothing in this field.
I have a couple of questions though:
1. Is all light a result of annihilation radiation?
2. What makes an electron an electron and a proton a proton if they carry the same kind of charge?
3. If photons have zero rest mass and the theory of relativity says energy and mass are convertible, can't we say photons have mass and what would the implication of that be?